Step by Step approach to achieve an FDA/MDIC approved Quality Management System

As the quote goes, “Many hands make light work.” (John Heywood, 1546)
This is very true with a Quality Management System, but not so if your QMS is still on paper. While there need to be several controls put in place plus various sets of eyes to review, handing paper from one set of hands to another creates inefficiencies and plenty of room for error. What happens when you lose those papers? Remember when “your dog ate your homework”? You don’t want that excuse when it’s time for your regulatory audit!
The items below are accurate for any industry, from medical devices, pharmaceuticals, biotech to manufacturing and marketing salt-related products. The FDA will want to see everything to safeguard the consumer and see that their guidelines, MDIC, and GMP ISO 9001 regulations are being met.
What are the recommended approaches to achieving an electronic FDA/MDIC approved QMS? I’m happy you asked:
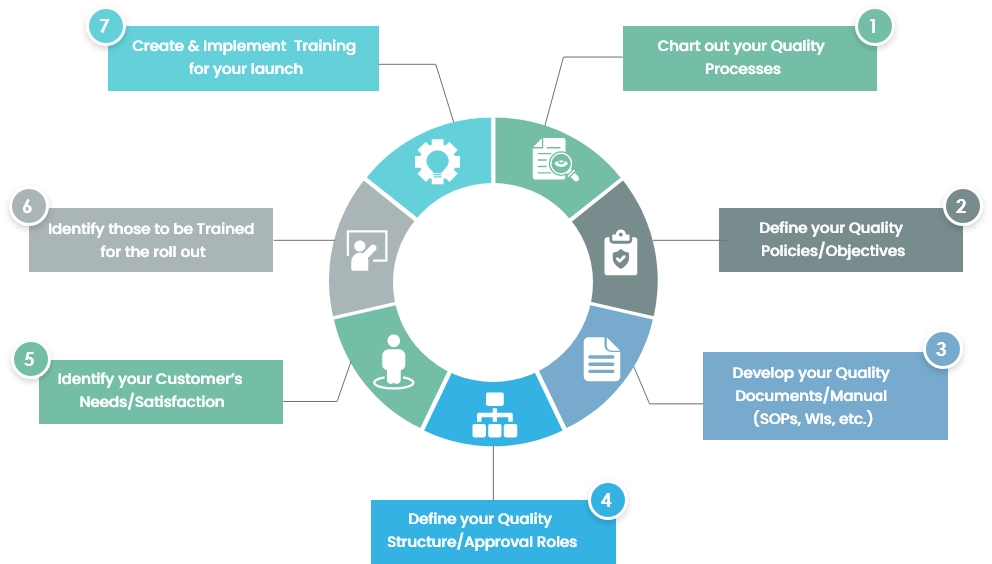
Setting these steps up will not only keep your company organized, create efficiencies and keep your problem-free with pending audits, but it will also keep you ahead of the competition, significantly ahead of those who don’t have an electronic QMS set up.
For instance, when a medical device is being developed, it is essential to follow the FDA’s particular guidelines. These conditions mandate a process for product development that is performed in sequential phases. Here, it is necessary to have completed one phase before the producer progresses to the next. This regulation guarantees that evaluating the risk and strength of the device is examined and recorded at every stage.
The tests for validating that the device performs as envisioned depend upon the user’s requirements in a scenario resembling real life. The product should be developed so that the place where it was made can be utilized to certify it. Suppose the outcome of the certification warrants a change in design. In that case, the producer must return to the drawing board to evaluate the strategy and production regulations before the product validation process is performed again. Most of the project phases require being examined and assessed, even if the changes in design are negligible or do not mitigate risk.
Connect with Compliance Group today at sales@complianceg.com or call (847) 327-3167, Ext. 406.