CG SUPPORTS AN END-TO-END SaMD LIFECYCLE PROCESS
CG expert resources provide consulting for a full QMS setup or execution of processes for requirements management, design, development, verification and validation, deployment, maintenance, and decommissioning of the product.

BLOG
What is the FDA’s approach to regulating AI/ML in SaMD (software as a medical device)?
According to the FDA’s site, their first Medical Device approved was in 1976 through their CDHR division per their 21CFR Parts 800-1299. As we know, medical devices fall into three categories from low risk to high risk
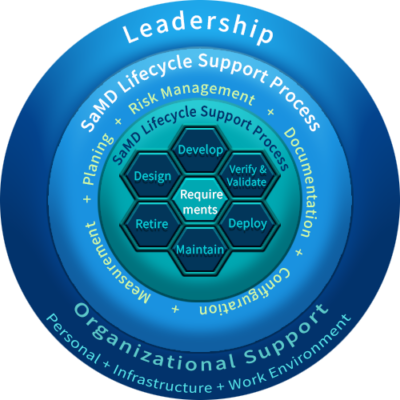
THE SaMD QUALITY MANAGEMENT SYSTEM
SaMD manufacturers are required to implement a Quality Management System setup based on best practice principles shown in the figure. CG sets up lifecycle support processes that are scalable for the size of the organization and ensures a consistent application of all processes.


Choosing Compliance Group CG to bring our SaMD product to market has proven to be an outstanding choice. Their inventive strategies, high end resources, skilled professionals, and profound comprehension of the SaMD lifecycle processes and regulatory prerequisites have propelled our digital health solutions to new heights. We appreciate a partnership that not only guarantees compliance but also nurtures innovation.
– Chief Medical Device Development Officer, Leading Medical Device Manufacturer
UNDERSTANDING SaMD AND SiMD
What is SaMD?
By definition, Software as a Medical Device (SaMD) is an application intended for medical use without use of hardware (International Medical Device Regulators Forum (IMDRF)). It is not software (like embedded software) fixed inside of a device. SaMD may include software that can control/interfere with the hardware and/or medical device and also mobile/wearables that meet the IMDRF definitions.
What is SiMD?
The Software in Medical Device (SiMD) category is often confused with SaMD and has different pathways. Generally, software that helps run a medical device is SiMD. However, many SiMD applications share characteristics of SaMD – for example, a mobile app, which is almost always associated with SaMD and can actually be SiMD.

